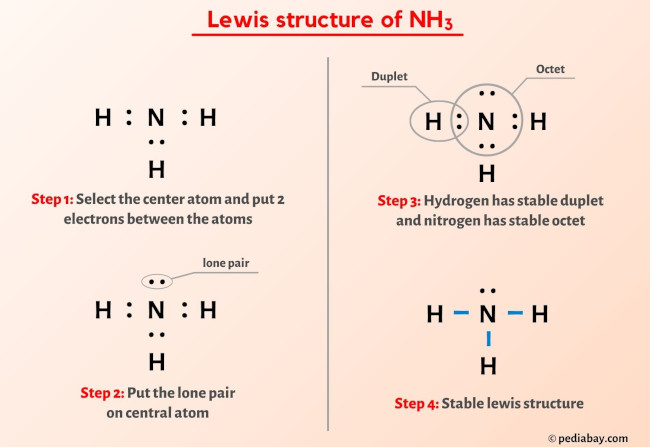
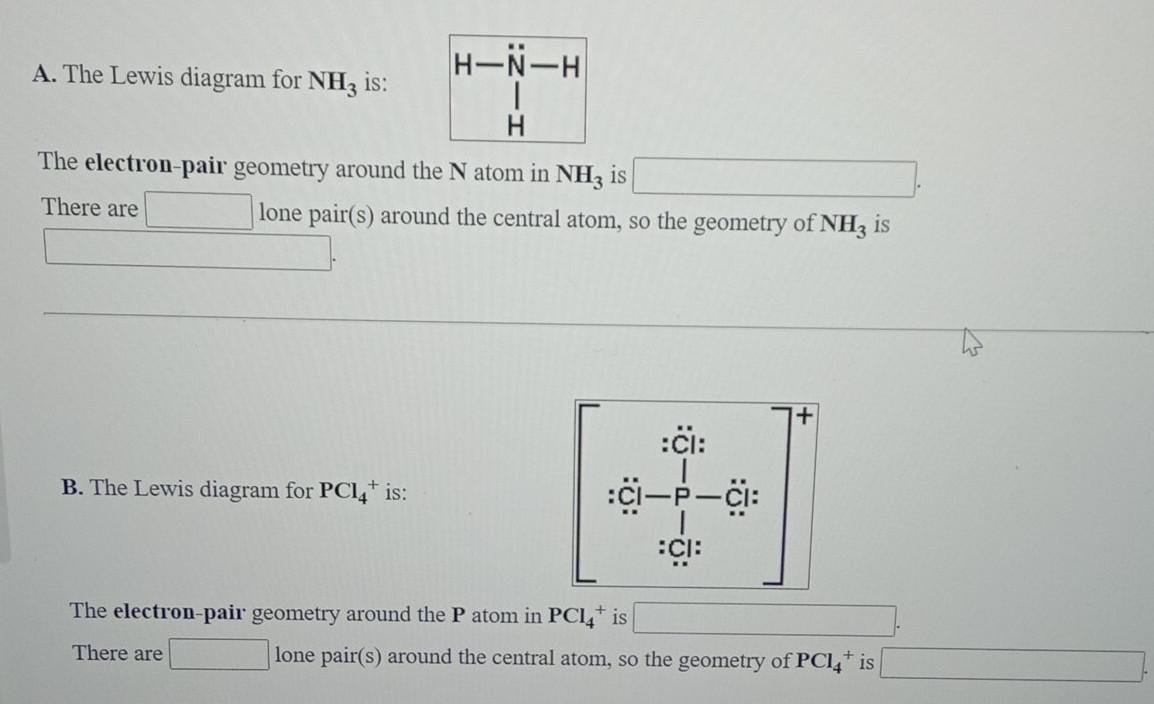
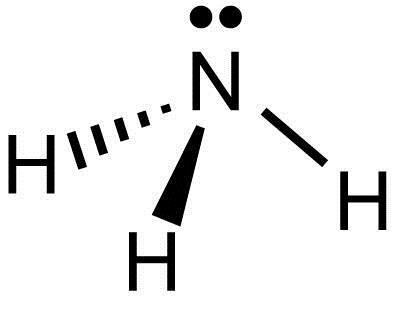
Draw the best Lewis structure for NH3 by filling in the bonds, lone pairs, and formal charges. (Assign - brainly.com
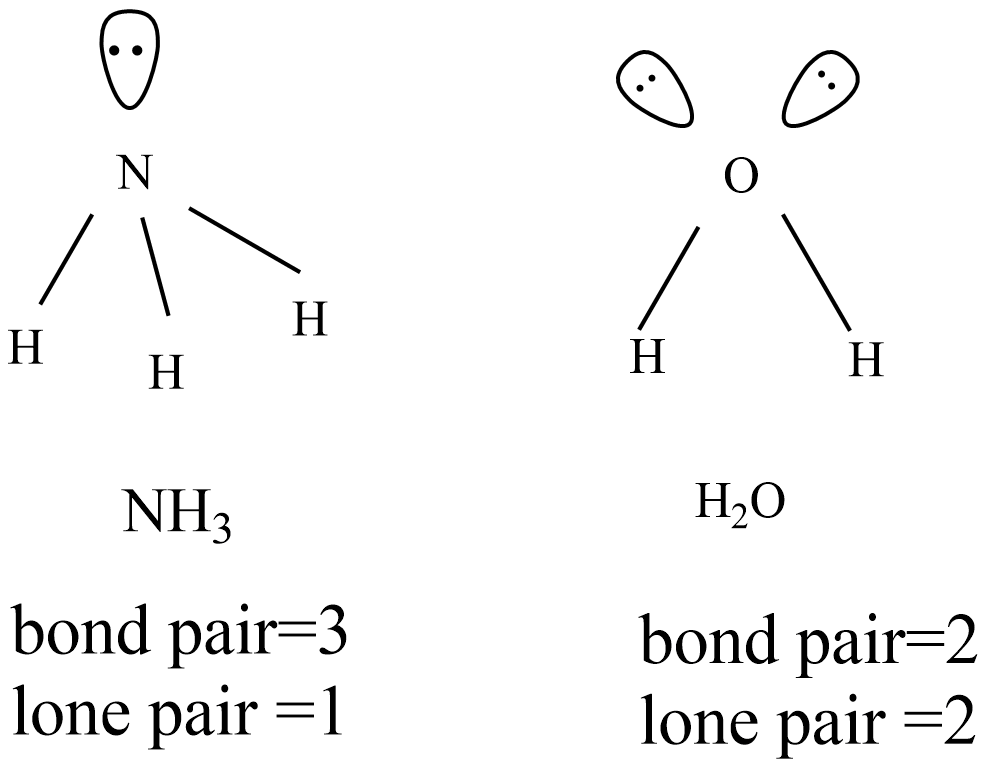
Why bond angle in water is less than that of ammonia although their geometries are distorted tetrahedral?

What do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one example of each type.

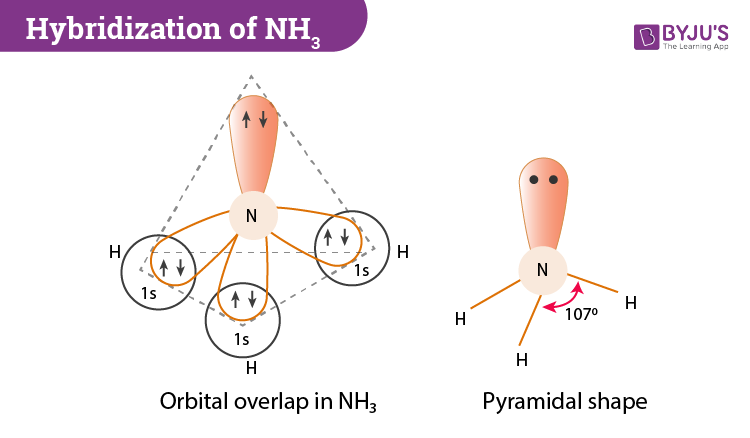
When correctly drawn, the Lewis dot structure for NH3 should have a lone pair of electrons on the central N. True False | Homework.Study.com



![Malayalam] Explain the bond pair electron and lone pair electrons H2O Malayalam] Explain the bond pair electron and lone pair electrons H2O](https://static.doubtnut.com/ss/web-overlay-thumb/5871017.webp)