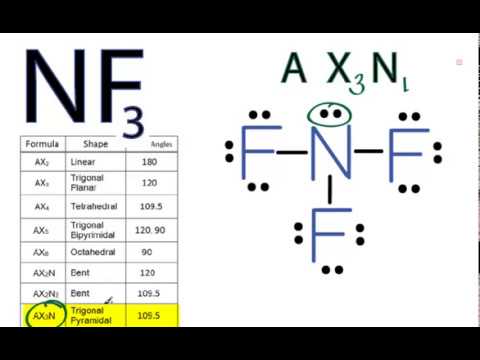
NF3 Lewis Structure (Nitrogen Trifluoride) | NF3 Lewis Structure (Nitrogen Trifluoride) Were you searching for a short yet detailed video on NF3 Lewis Structure? If yes then we have got you. Today...

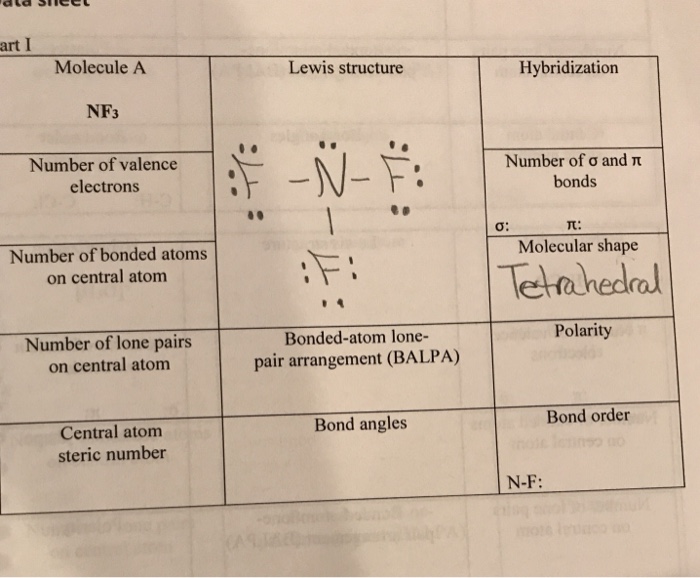
write lewis structures of nf3. what is the electronic and molecular geometry? is the molecule polar or - brainly.com

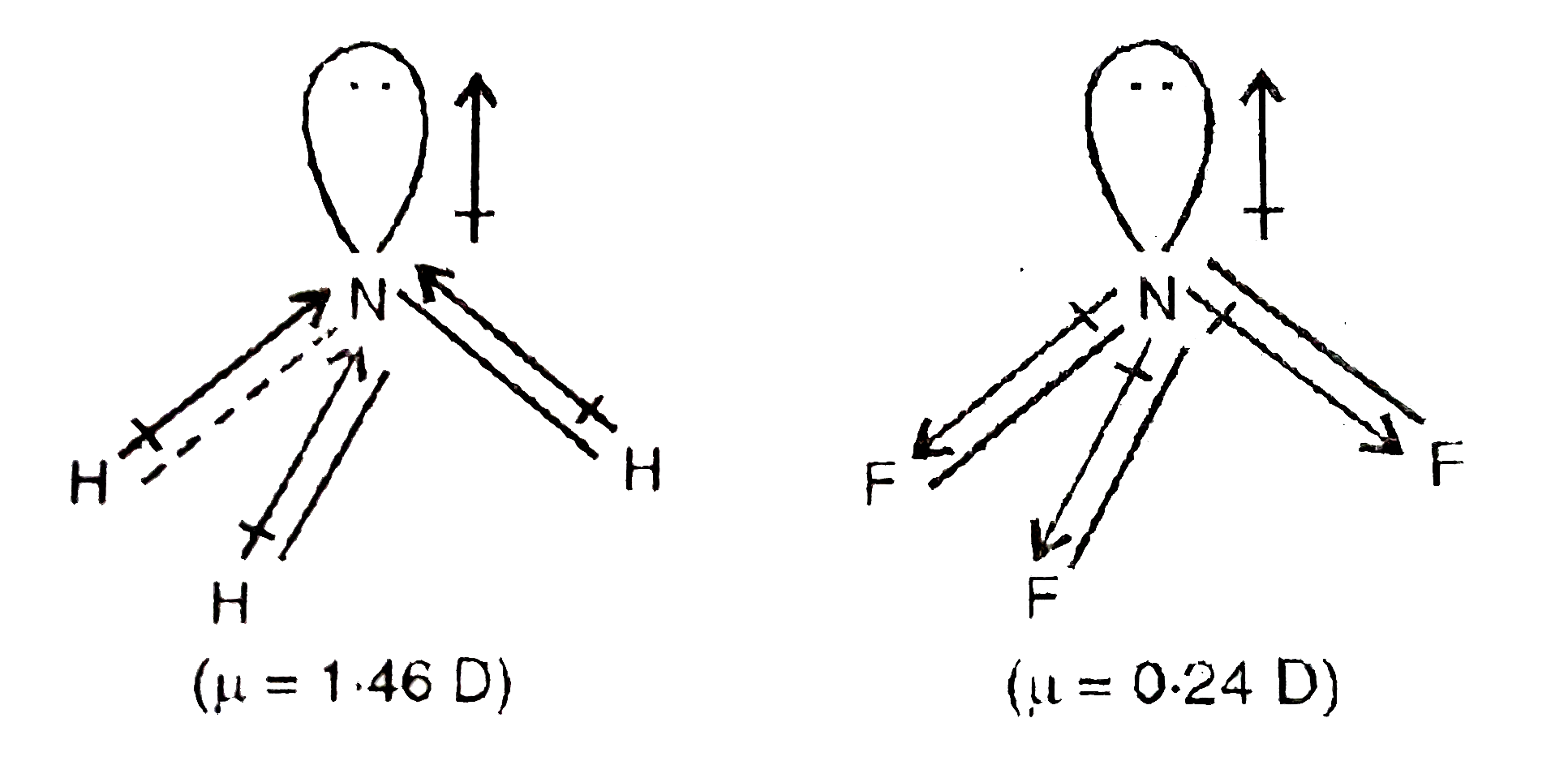
BF_{3} and NF_{3} both are covalent compounds but NF_{3} is polar whereas BF_{3} is non-polar. This is because :
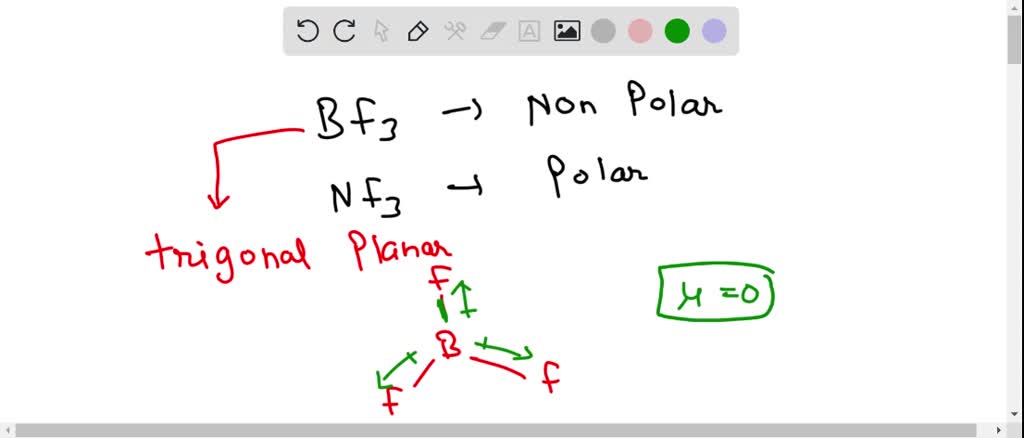
SOLVED: 41. The BF3 molecule is nonpolar, whereas the NF3 molecule is polar. Which of the following statements accounts for the difference in polarity of the two molecules? In NF3, each F

Is NF3 Polar or Nonpolar? - Polarity of Nitrogen trifluoride | Molecular geometry, Polar, Covalent bonding
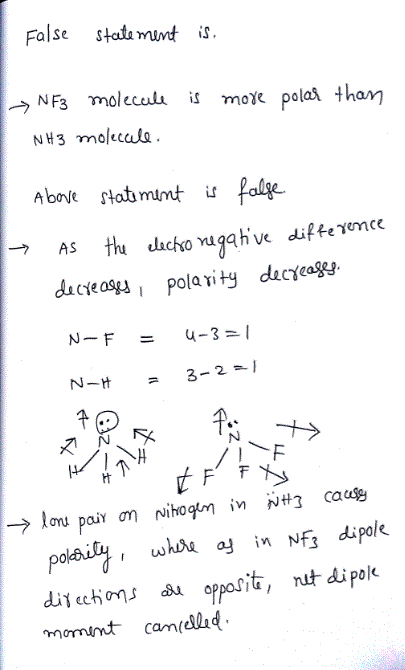
Which statement for NH3 and NF3 is false? Electro negativities N = 3.0, H = 2.1, F = 4.0 - Home Work Help - Learn CBSE Forum

![ANSWERED] A Is CH O polar or nonpolar B Is NF3 polar... - Organic Chemistry - Kunduz ANSWERED] A Is CH O polar or nonpolar B Is NF3 polar... - Organic Chemistry - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20220502230749422436-4415097.jpg)