IRISH MEDICINES BOARD ADDITIONAL MONITORING: BLACK TRIANGLE INTRODUCED AS PROMPT TO REPORT SUSPECTED ADVERSE REACTIONS

EU Commission Report on National and EMA experience on Medicines subject to Additional Monitoring released today
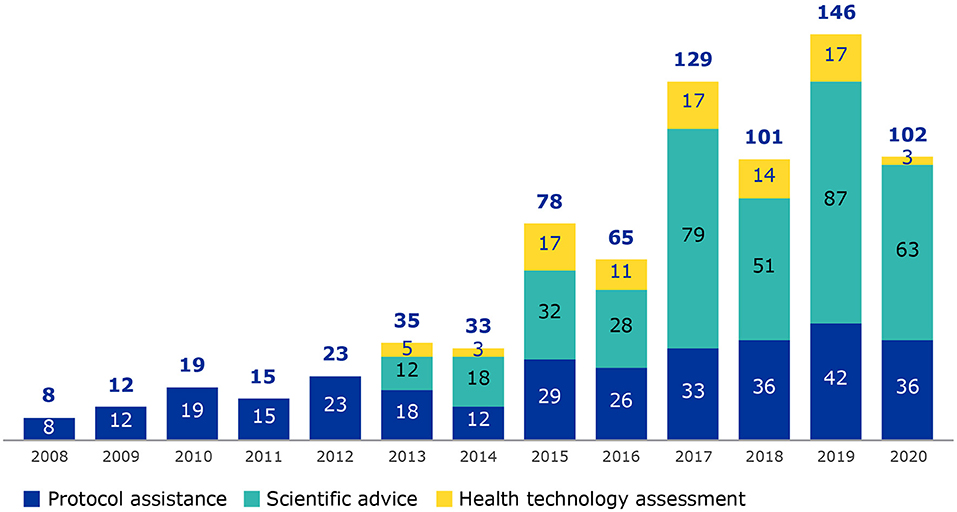
Frontiers | The Added Value of Patient Engagement in Early Dialogue at EMA: Scientific Advice as a Case Study

EMA updates SmPC and package leaflet templates with new black symbol | Signs & Symptoms of Translation

Evaluation of quantitative signal detection in EudraVigilance for orphan drugs: possible risk of false negatives | Semantic Scholar

PDF) Does additional monitoring status increase the reporting of adverse drug reaction s ? An interrupted time series analysis of EudraVigilance data

Monitoring evidence on overall survival benefits of anticancer drugs approved by the European Medicines Agency between 2009 and 2015 - ScienceDirect